
123
New drug creation, such as YB1 thrombolysis, offers new hope for the treatment of cardiovascular disease as medical costs steadily rise.
Over the past 30 years, the prevalence of cardiovascular disease and mortality among Chinese residents has gradually increased, and now cardiovascular disease has become the first cause of death in China; of the 330 million people currently suffering from cardiovascular disease, the number of people with coronary heart disease is about 11 million, while the mortality rate of patients with acute myocardial infarction remains high and continues to rise …
The absolute number of people suffering from hypertension, dyslipidaemia, diabetes, and obesity in China is still rising due to the widespread prevalence of lifestyle risk factors such as unhealthy diet, physical inactivity, and smoking, further pushing up the morbidity mortality rates of cardiovascular diseases in the country.
To this end, the National Cardiovascular Centre and other official bodies have said that it is essential to adhere to the concept of “greater health, greater health” and to move away from focusing only on the risk factors themselves to the environment in which they are formed and prevalent, and to pay more attention to the role of environmental factors and lifestyles in the formation of risk factors.
In China, the prevention and treatment of cardiovascular diseases, emphasizing cardiovascular health, adopting a strategy that focuses on zero-level prevention of hypertension, dyslipidemia, diabetes, obesity and smoking, integrating health into all policies and forming a social environment conducive to a healthy lifestyle are the only ways to promote a shift from a treatment-centred approach to a health-centred approach and improve the health of the people.
As cardiovascular prevention and treatment of cardiovascular disease have become a national concern, fundamental research into related diseases, the manufacture of medical devices, and the development of innovative drugs have also picked up speed, creating a glowing blue ocean market.
Accelerated basic research and device approval for cardiovascular disease lay the foundation for industrial development.
Reviewing the history of the development of the cardiovascular disease research field in China, high-level basic cardiovascular research in mainland China began to take off after 2005, with influential papers published mainly in the two influential journals, Circulation and circles. In recent years, the rapid development of high-level basic cardiovascular research in China can be observed through data from journals such as Circulation, JAmCollCardiol, EurHeartJ and NatCommun.
Publicly available information shows that from 2019 to 2020, there are 58 essential research papers with both corresponding and lead authors from mainland China aimed at exploring the anatomy, development and function/pathogenesis of the heart and blood vessels. According to the research directions, there are 46 studies related to cardiac pathology and 12 studies in the vascular field, covering myocardial diseases (ischemic heart disease, cardiomyopathy, myocarditis, heart failure, etc.), cardiac arrhythmias, atherosclerosis, and growth and development.
Amongst the topical studies are the integration of single-cell sequencing technologies into cardiovascular development and disease, cardiomyocyte development and regeneration, the role of cardiac inflammatory cells in development and disease progression, and gene therapy.
From September 1, 2019, to August 31, 2020, the State Drug Administration approved 39 medical devices to enter the innovative medical device review channel, of which 12 were cardiovascular products, indicating that innovation in the cardiovascular field dominates the medical device innovation field in China, accounting for 30.8%; and there were 34 original domestic products, accounting for 87.2%.
From September 1,2019 to October 22, 2020, the State Drug Administration approved 141 registration certificates for three types of medical devices in the cardiovascular field, of which 96 were domestic products and three products entered the national innovative medical device review channel. Among these 96 domestic products, there were 69 interventional products, 6 active surgical products containing tablets, 5 emergency products, 4 diagnostic products, 4 imaging products, 2 open surgery products, 3 AI software, 1 blood flow measurement system, 1 extracorporeal circulation device and 1 other device.
From the number of approved provinces and municipalities that can be seen, the current geographical distribution of cardiovascular three types of medical devices still varies greatly, with Guangdong Province ranking first, with a total of 28 product registrations in a year, followed by Shanghai, Jiangsu and Beijing. At the same time, there are still more than 20 provinces and municipalities directly under the Central Government to obtain the number of certificates “zero”.
Domestic drug companies ramp up the layout of original new cardiovascular drugs, bringing new hope for treatment.
With the advancement of modern technology, several original new cardiovascular drugs in China have gained good evidence of efficacy and safety in clinical studies and practice in recent years, bringing new hope for the treatment of cardiovascular diseases.
For example, the international multi-centre Phase II clinical study of the heart failure treatment drug Istaroxime has been completed. The international multi-centre Phase III, clinical study protocol of the heart failure treatment drug recombinant human Neurontin (rhNRG-1), has been granted fast-track status by the FDA, and the Chinese Phase III clinical study is in progress. Meanwhile, levosimendan, a positive inotropic drug, has been marketed, and a Phase IV clinical study in China is in progress; Recombinant human cerebral natriuretic peptide, a treatment for acute heart failure, has been marketed. The Phase III clinical study of vincristine, an anti-platelet drug, is in progress in China; And alisartanate, a treatment for hypertension, has also been marketed.
In addition, outside the framework of existing guideline-oriented drug therapy, the use of traditional medicine for the treatment of cardiovascular disease has been explored, such as Astragalus yiqi drops, Heart Pulse Long injection, Ginseng Yangxin capsule, Astragalus hebecarpa capsules, Compound Dan chin drops and Tongxinluo, some of which have achieved new evidence-based medical evidence.
Starting from the enthusiasm of new players in the industry, more and more biotechnology companies have been involved in the research and development of new drugs in the field of cardiovascular therapeutics in recent years For example, HKND, which has YB1, a bio molecular drug delivery vehicle, as its core technology product, has started to lay out an innovative thrombolytic drug product pipeline, and the relevant thrombolytic drugs under development will be applied to the treatment of various thrombotic diseases which belong to the scope of cardiovascular disease treatment.
Our self-developed bio molecular drug delivery vehicle YB1 can be combined with a variety of thrombolytic drugs to treat various thrombotic diseases. We have three pipelines of thrombolytic drugs under development, namely YB1-rt-PA, YB1-rt-DE and YB1-rt-PL, and the clinical effects of thrombosis treatment are worthy of anticipation.
Cardiovascular therapeutic end markets look promising as healthcare costs steadily rise.
Data from industry research reports show that the total number of discharges of patients with cardiovascular and cerebrovascular diseases from Chinese hospitals in 2018 was 23,161,300, accounting for 12.8% of the total number of discharges (including all inpatient conditions) in the same period; among them, the total number of discharges for cardiovascular diseases was 11,423,900, accounting for 6.31%, and for cerebrovascular diseases was 11,737,400, accounting for 6.48%.
The following graph shows the trend in the number of hospital discharges for patients with cardiovascular disease in China from 1980 to 2018.
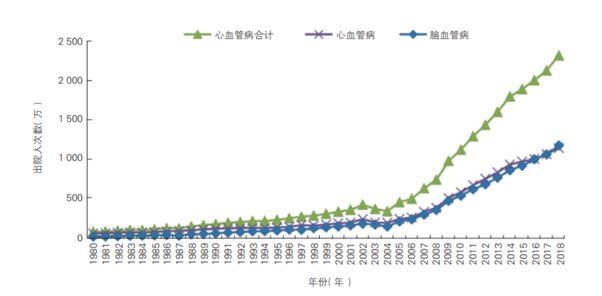
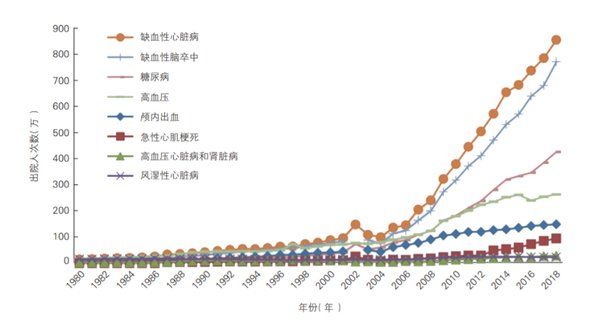
Of all cardiovascular discharges, the highest proportions of the population were for ischaemic heart disease (8,588,800, of which 952,100 were for acute myocardial infarction) and ischaemic stroke (7,723,400), with shares of 36.95% and 33.35%, respectively.
From 1980 to 2018, the average annual growth rate of hospital discharges for cardiovascular diseases in China was 9.73%, faster than the average annual growth rate of hospital discharges for all diseases in the same period (6.34%). The top three diseases in terms of average annual growth rate were ischemic stroke death (12.03%), ischemic heart disease (11.22%) and acute myocardial infarction (10.94%). The average annual growth rate of diabetes discharges from 1980 to 2018 was 13.45%.
The following graph shows the trend in the number of hospital discharges for each significant cardiovascular disease and diabetes type in China from 1980 to 2018.
In terms of data related to medical costs, the information shows that in 2018, the total cost of hospitalisation for ischaemic heart disease amounted to RMB 111,982 million, including RMB 23,567 million for acute myocardial infarction; RMB 65,432 million for ischaemic stroke and RMB 27,072 million for intracranial haemorrhage (see chart below.; RMB 16,667 million for hypertension. Furthermore, RMB 33,172 million for diabetes; net of the effect of price factors, since 2004, the average annual growth rates of inpatient costs for acute myocardial infarction, ischaemic stroke and intracranial haemorrhage were 26.89%, 18.65% and 14%, respectively.
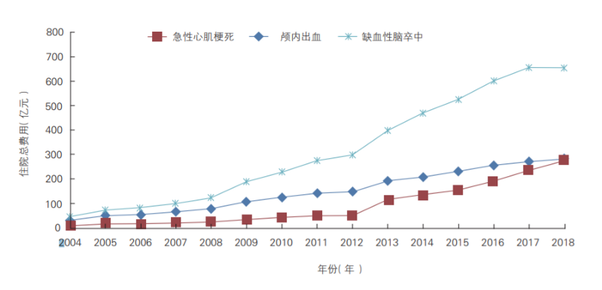
In 2018, the average sub-hospitalisation cost for cardiovascular diseases was $13,083.9 for ischaemic heart disease, including $28,879.3 for acute myocardial infarction; $9,409.64 for ischaemic stroke and $18,863.63 for intracranial haemorrhage; $6,322.54 for hypertension; And $ 7,773.9 for diabetes mellitus; net of the effect of price factors, since 2004 The average annual growth rates of the sub-average hospitalisation costs for acute myocardial infarction, ischaemic stroke and intracranial haemorrhage were 6.09%, 1.26% and 4.73% respectively.
It can be seen that in recent years, China's cardiovascular disease medical expenses have shown a steady rise, suggesting that the end market in the field of cardiovascular disease treatment is vast and optimistic. The whole industry still has considerable room for growth in the future, expecting new biotechnology companies to bring more choices for the majority of cardiovascular disease patients with more innovative research results and stimulate the development of the industry in the competition with more vitality.
喜欢我的文章吗?
别忘了给点支持与赞赏,让我知道创作的路上有你陪伴。
发布评论…