
123
Focus on reperfusion therapy: Leading players lead thrombolysis track, emerging biotech companies accelerate the layout?
With the expansion of the social economy and the acceleration of urbanization, people's material living standard has been greatly improved. More and more people have become accustomed to living in the jungle of steel and concrete. Although people's living standards have been greatly improved in terms of material aspects, people's bodies seem to be more "vulnerable": In the better life today, several increasingly frequent diseases also bring great trouble to human health.
Among the various health threats, Cardiovascular disease is one of the most serious health hazards that contemporary individuals confront. Myocardial infarction (caused by blockage of arterial blood vessels), stroke (commonly known as “Cerebrovascular event”), acute pulmonary embolism, and other cardiovascular diseases caused by blocked arteries claim many lives each year and even become the largest cause of death in the worldwide. In the therapy of such diseases, we often hear the word “reperfusion therapy”, but many people don’t know what it is all about.
In fact, reperfusion therapy is an important method of intervention therapy for cardiovascular diseases. It is a method to completely occluded vessels are reopened and ischaemic tissue is reperfused through thrombolytic drugs, interventional therapy, or surgical treatment. In many cases, reperfusion therapy is a life-saving key operation for patients with cardiovascular emergencies. Among them, thrombolytic therapy has become more and more common in clinical applications.
Acute ischemic stroke and how to treat it
Stroke is one of the most common cardiovascular diseases in China after hypertension. Stroke is an acute cerebrovascular disease, mainly including ischemic stroke and hemorrhagic stroke. It is a kind of disease caused by the sudden rupture of blood vessels in the brain or blockage of blood vessels that the inability of blood to flow to the brain causes damage to brain tissue, and is characterized by high morbidity, mortality, and disability.
Stroke is currently dominated by acute ischemic stroke (AIS), which accounts for about 70.2% of all stroke cases. Publicly available information records that the number of deaths due to acute ischemic stroke in China in 2019 was approximately 719,500. Meanwhile, It has also been shown that the rate of death/disability within one year of symptom detection in hospitalized acute ischemic stroke patients ranges from 33.4% to 33.8%.
According to the Chinese Guidelines for the Diagnosis and Treatment of Acute Ischemic Stroke, the main treatment for acute ischemic stroke is currently thrombolysis.
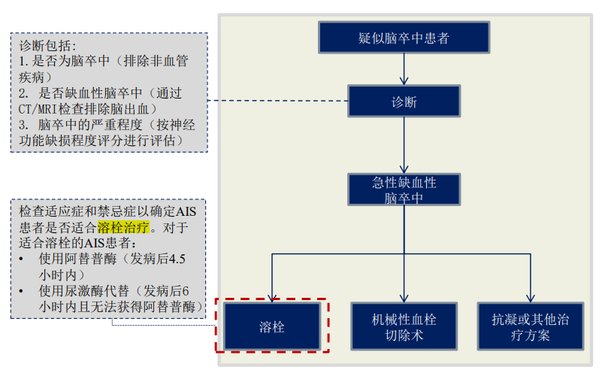
According to Frost & Sullivan’s report, around 4.832 million people in China would suffer from stroke in 2019, with approximately 3.3921 million suffering from acute ischemic stroke. Due to the growing prevalence of hypertension, diabetes, hyperlipidemia, and coronary heart disease, China’s acute ischaemic stroke population is anticipated to reach approximately 3,977,100 by 2024 and 4,342,400 by 2030.
The time crucial for treating acute ischemic stroke therapy is particularly important due to the lack of blood supply to the brain after the stroke onset and the subsequent brain damage, which results, in turn, leads to functional following impairment after acute ischaemic stroke treatment.
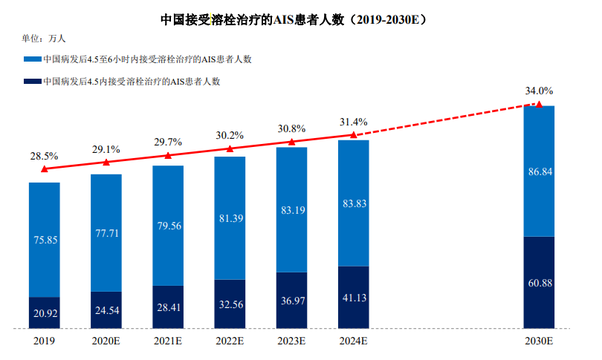
Of all acute ischemic stroke patients treated in China in 2019, only about 16.0% had access to treatment within 4.5 hours of onset, while about 58.0% had access to treatment between 4.5 and 6 hours after onset. Despite the increasing awareness of acute ischemic stroke and the increasing availability of medical resources, it is still difficult for many acute ischemic stroke patients to reach a hospital within a short time of onset.
In 2019, the number of patients with acute ischemic stroke who could receive thrombolytic therapy in China was approximately 1,307,700, and the number of patients who could be suitable for thrombolytic drug treatment within 6 hours of onset was approximately 967,700, of which only approximately 758,500 patients could receive thrombolytic therapy between 4.5 hours and 6 hours.
The number of patients with acute ischemic stroke who can get thrombolysis within six hours is expected to rise to 1,249,600 in 2024, owing to favorable policies such as the future in medical resources expansion and the construction of stroke centers.
Policy support and increased accessibility to reperfusion therapy
To coordinate medical resource allocation, the Chinese government at all levels has continued to encourage the development of a graded diagnostic and treatment system, depending on the majority of hospitals and primary health care institutions to do so. China has recently issued a series of policies to promote the treatment of stroke and heart attack patients in China, including the Notice on the Issuance of Guidelines for the Construction and Management of Chest Pain Centers (for Trial Implementation) and the Notice on Further Strengthening Stroke Treatment Management to promote the treatment of stroke and heart attack patients in China.
With China vigorously supporting the construction of chest pain centers and stroke centers, the time between pre-hospital emergency and hospital treatment for patients has been gradually shortened, the efficiency of thrombolysis for patients has been improved and the accessibility of patients receiving reperfusion therapy has been further enhanced. Statistics show that by the end of 2019, there were more than 1,200 units of state-certified chest pain centers and more than 4,300 registered hospitals nationwide.
At the same time, the state has also introduced relevant policies to vigorously support the development of more innovative drugs.
To accelerate the development of China’s innovative drug industry, the State has issued a series of policies covering priority evaluation and subsequent reimbursement by medical insurance, from drug registration to end-use of drugs to support innovative drugs. With the effective establishment of a dynamic adjustment mechanism for the scope of drugs used in basic medical insurance, more innovative drugs will have the opportunity to be added to the national medical insurance catalog in the future, helping the development of innovative drugs from an economic perspective.
Competitive overview of the thrombolytic therapy industry: Leading enterprise to lead the way, with emerging biotech companies accelerating their layout
Driven by the continuous growth of market demand and active support from national policies, cardiovascular disease treatment, antithrombotic drug treatment, and the more subdivided thrombolytic drug treatment fields are gradually ushering in a climax of development and competition.
Overall, the competitive track in the thrombolytic therapy segment is still continuously led by the old leading companies, but also ushers in the accelerated layout of many new biological companies, injecting fresh blood and promising momentum for the long-term development of the industry.
In addition to Tianshi Li, whose core product is Puyuk, which ranks third in the domestic thrombolytic drug market, other established companies in the industry include Boehringer Ingelheim, a company founded in 1885 and established in China in 1995 as Boehringer Ingelheim Shanghai Pharmaceutical Co. The company’s main business areas include immune and respiratory diseases, cardiovascular and metabolic diseases, central nervous system diseases, and oncology. Its alteplase, which was approved for marketing in China in 2002, is currently used in the treatment of acute myocardial infarction, pulmonary embolism, acute ischaemic stroke, deep vein thrombosis, and other vascular diseases.
And CHINA RESOURCES ANGDE BIOTECH PHARMA CO., LTD was established in 2001, mainly engaged in the production and sales of biological agents. Its main products are recombinant human erythropoietin injection (CHO cells), recombinant human interleukin-11(I) for injection and recombinant human tissue-type fibrinogen kinase derivatives for injection. The company’s recombinant human tissue-type fibrinogen kinase derivative for injection was approved for marketing in 2007, mainly for the treatment of acute myocardial infarction, and is a product on the current national medical insurance list (Class B).
In addition, RECOMGEN BIOTECH, Guangzhou RECOMGEN BIOTECH CO., LTD.was established in 2000 and is engaged in the research, development, production, and sales of new biotechnology drugs. The company’s main product is a recombinant human TNK tissue-type fibrinogen activator, which was approved for marketing in 2015 and is mainly used for the thrombolytic treatment of patients with acute myocardial infarction within 6 hours of onset.
As an emerging biotechnology company, we are currently developing several innovative thrombolytic drugs at HKND YB1 PHARMACEUTICAL Using our self-developed biomolecular drug delivery vehicle, YB1, in combination with a variety of thrombolytic drugs, our thrombolytic target products can treat a variety of thrombotic diseases.
Currently, HKND YB1
PHARMACEUTICAL Thrombolysis has laid out three pipelines of thrombolytic drugs under development, namely YB1-rt-PA, YB1-rt-DE, and YB1-rt-PL, of which YB1-rt-PA is the company’s first-generation targeted thrombus ablation product. The main feature is that it is carried by YB1 and releases Urokinase (Urokinase) at the site of the thrombus.
With more emerging biotech companies like HKND YB1 PHARMACEUTICAL actively setting up, the reperfusion therapy and thrombolysis track is becoming more and more lively, and look forward to a whole new era in the development of the entire cardiovascular disease treatment field.
喜欢我的文章吗?
别忘了给点支持与赞赏,让我知道创作的路上有你陪伴。
发布评论…